Redefining HER2 Detection: Precision Proteomics Illuminates Hope for Ultra-Low Expressing Breast Cancer
ยาความแม่นยำ
•
2025-11-07
The therapeutic landscape of HER2-positive breast cancer continues to evolve—from positive to low and now ultra-low expression categories. Trastuzumab deruxtecan has redefined HER2-targeted therapy, extending clinical benefit to broader patient populations. This evolution, however, underscores the need for greater precision and consistency in HER2 assessment. Mass spectrometry-based clinical proteomics provides an advanced, quantitative, and reproducible solution that complements conventional IHC, enabling more accurate patient stratification and guiding the next generation of precision oncology.
Original article from: mProbe

1. DESTINY-Breast06 Study: Advancing HR-Positive Advanced Breast Cancer Treatment
The boundaries of HER2-targeted therapy in breast cancer—ranging from positive, low expression to ultra-low expression—are continuously being redefined. On January 27, 2025, the U.S. Food and Drug Administration (FDA) expanded the indication for trastuzumab deruxtecan (Enhertu, fam-trastuzumab deruxtecan) to include patients with hormone receptor-positive (HR+), HER2 ultra-low expression (IHC 0 with membrane staining) advanced breast cancer who has progressed on endocrine therapies. Initially approved in December 2019 for HER2 positive, unresectable, or metastatic breast cancer, trastuzumab deruxtecan’s approval was expanded in August 2022 to include HER2-low expression. The latest approval now extends its use to HER2 ultra-low expression patients.
This expanded indication was supported by data from the phase III DESTINY-Breast06 clinical trial, presented at the 2024 ASCO Annual Meeting and simultaneously published in the New England Journal of Medicine.1,2 Following the groundbreaking DESTINY-Breast04 trial in 2022, which opened a new era for HER2-low expression breast cancer treatment³, the 2024 DESTINY-Breast06 trial further redefined therapeutic boundaries by demonstrating efficacy in HER2 ultra-low expression. HER2 ultra-low expression has now emerged as a new diagnostic and therapeutic focus in breast cancer, bringing forth new challenges for pathologists in accurately identifying this category.
This article will walk you through the groundbreaking data redefining breast cancer standards, review the evolution of HER2-targeted therapy, discuss the clinical significance of HER2 low/ultra-low detection, and introduce a powerful new tool—mass spectrometry-based absolute quantitative proteomics—for precise HER2 assessment.
2. The Evolution of HER2-Targeted Therapy in Breast Cancer
Historically, based on human epidermal growth factor receptor 2 (HER2) protein expression, breast cancer was classified into two groups: HER2-positive and HER2-negative. Approximately 15–20% of breast cancers exhibit HER2 overexpression and are categorized as HER2-positive. Tumors not classified as HER2-positive were considered HER2-negative. The HR-positive, HER2-negative subtype represents about 70% of all breast cancers. Endocrine therapy is commonly used as first-line treatment for HR-positive metastatic breast cancer; however, therapeutic benefit diminishes after initial response, leaving chemotherapy as the current standard post-endocrine therapy. 4,5,6
Based on trastuzumab deruxtecan trial data, experts have identified two additional HER2 expression categories: HER2-low and HER2-ultra-low. HER2-low refers to immunohistochemistry (IHC) 1+ or 2+ with negative in situ hybridization (ISH–), while HER2-ultra-low is defined as IHC 0–1+, characterized by incomplete, faint membrane staining in >0% and ≤10% of tumor cells.
3. Why Refining HER2 Classification Matters: A Timeline of Anti-HER2 Therapy
Early HER2-targeted therapies such as trastuzumab (Herceptin) and pertuzumab (Perjeta) act like precision-guided antibodies that bind to extracellular domains of HER2. Later-generation small-molecule inhibitors such as lapatinib (Tykerb), neratinib (Nerlynx), and tucatinib (Tukysa) target the intracellular tyrosine kinase domain of HER2, preventing receptor autophosphorylation and suppressing aberrant signaling cascades driven by HER2 activation. However, these drugs were mainly effective against HER2-positive tumors and offered limited benefit for those with minimal HER2 expression. As a result, HER2-low and HER2-ultra-low tumors had long been classified as HER2-negative.
Newer-generation therapies such as trastuzumab deruxtecan employ a dual mechanism—precise HER2 targeting and intracellular delivery of cytotoxic payloads through an antibody-drug conjugates (ADC) design. Upon binding to HER2 on the tumor cell surface, the ADC is internalized, where it releases its payload to induce targeted cell death. This breakthrough has redefined the HER2 landscape, leading to the reevaluation of tumors once deemed HER2-negative to identify those that are actually HER2-low or ultra-low and potentially responsive to this new therapeutic class.
4. Determining HER2 Status: Diagnostic Criteria and Challenges
HER2 status is assessed using tumor tissue obtained from surgical or biopsy specimens. Pathologists perform immunohistochemistry (IHC) to evaluate HER2 protein expression on a 0–3+ scale: 0 indicates no detectable staining, while 3+ represents strong, complete membranous staining in >10% of tumor cells. Tumors with an IHC score of 2+ are considered equivocal and require confirmatory fluorescence in situ hybridization (FISH) testing to assess HER2 gene amplification.
HER2-low tumors are defined as IHC 1+ or 2+ with FISH– results, whereas tumors with IHC 0 may be further subclassified into HER2-ultra-low (detectable faint/incomplete membrane staining in ≤10% of tumor cells) or HER2-negative (no staining).
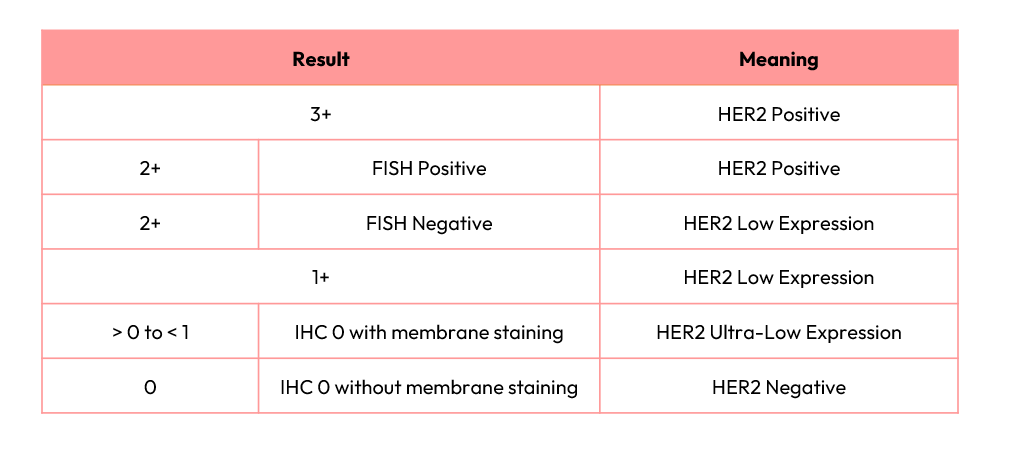
5. Clinical Implications: Pathological and Diagnostic Challenges
In the DESTINY-Breast06 study, HER2 IHC results required central laboratory confirmation for eligibility. For HER2-ultra-low enrollment, patients had to be locally diagnosed as IHC 0 but centrally confirmed as between 0 and 1+. This subtle distinction between true IHC 0 (no membrane staining) and HER2-ultra-low presents significant interpretive challenges for pathologists.
Exploratory analyses revealed that 64% of tumors initially scored as IHC 0 by local laboratories were reclassified by central review as HER2-low (24%) or HER2-ultra-low (40%). These findings suggest that HR-positive metastatic breast cancer patients initially labeled as IHC 0 should be reconsidered for potential eligibility for trastuzumab deruxtecan therapy.7
6. The Power of Precision: Mass Spectrometry-Based Absolute Quantitative Proteomics
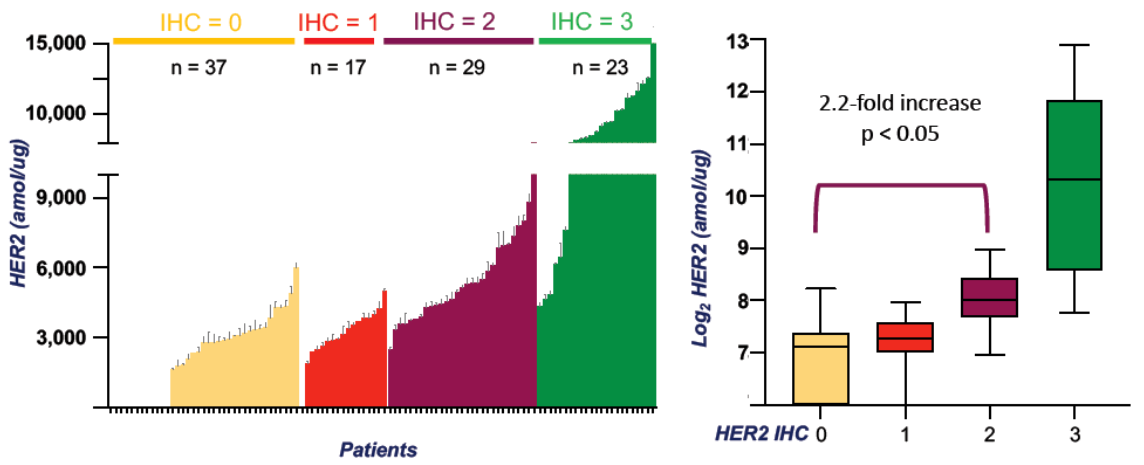
HER2 expression serves as a critical prognostic marker and predictor of anti-HER2 therapy response. As HER2 testing thresholds expands from high to low and now ultra-low, accurate quantification of HER2 protein becomes essential. Traditional IHC methods are semi-quantitative, antibody-dependent, and prone to inter-observer variability. Mass spectrometry-based absolute quantitative proteomics overcomes these limitations through he use of isotopically labeled internal standard peptides, enabling multiplexed, high-sensitivity, and high-specificity quantitation.
Using this method, 173 breast cancer samples representing various IHC categories were analyzed. HER2 concentrations correlated with IHC scores (lowest in IHC 0, highest in IHC 3+). Remarkably, 73% of IHC 0 samples exhibited measurable HER2 protein expression (168–623 amol/μg). HER2 levels were significantly higher in IHC 2+ versus 0 (p<0.05), while differences between 1+ and 2+ were not statistically significant.8
Conclusion
The therapeutic landscape of HER2-positive breast cancer continues to evolve—from positive to low and now ultra-low expression categories. Trastuzumab deruxtecan has redefined HER2-targeted therapy, extending clinical benefit to broader patient populations. This evolution, however, underscores the need for greater precision and consistency in HER2 assessment. Mass spectrometry-based clinical proteomics provides an advanced, quantitative, and reproducible solution that complements conventional IHC, enabling more accurate patient stratification and guiding the next generation of precision oncology.
Reference
- Giuseppe Curigliano et al., Trastuzumab deruxtecan (T-DXd) vs physician’s choice of chemotherapy (TPC) in patients (pts) with hormone receptor-positive (HR+), human epidermal growth factor receptor 2 (HER2)-low or HER2-ultralow metastatic breast cancer (mBC) with prior endocrine therapy (ET): Primary results from DESTINY-Breast06 (DB-06). JCO 42, LBA1000-LBA1000(2024)
- Bardia A, Hu X, Dent R, et al. Trastuzumab Deruxtecan after Endocrine Therapy in Metastatic Breast Cancer. N Engl J Med. 2024;391(22):2110-2122.
- Modi S, Jacot W, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med. 2022;387(1):9-20.
- Ahn S, et al. HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation. J Pathol Transl Med. 2020;54(1):34-44.
- Sajjadi E, et al. Improving HER2 testing reproducibility in HER2-low breast cancer. Cancer Drug Resist. 2022;5(4):882-888.
- Manohar P, et al. Updates in endocrine therapy for metastatic breast cancer. Cancer Biol Med. 2022 Feb 15; 19(2):202–212.
- Salgado RF, et al. LBA21 - Human epidermal growth factor receptor 2 (HER2)-low and HER2-ultralow status determination in tumors of patients (pts) with hormone receptor–positive (HR+) metastatic breast cancer (mBC) in DESTINY-Breast06 (DB-06). Annals of Oncology. (2024) 35 (suppl_2): 1-72. 10.1016/annonc/annonc1623.
- Fabiola C, et al. Quantitative mass spectrometry of HER2 protein levels reveals high variability within HER2 IHC grades [abstract]. In: Proceedings of the Annual Meeting of the American Association for Cancer Research 2020; 2020 Apr 27-28 and Jun 22-24. Philadelphia (PA): AACR; Cancer Res 2020;80(16 Suppl):Abstract nr 5128.









.jpg)
.png)

